Role:
Sole Product Designer
Client:
8 months
Product Type:
Enterprise B2B SaaS
Pathology labs today generate thousands of high-resolution images per study, yet many teams still rely on Excel sheets, shared drives, and manual cross-referencing to manage critical research data.
ATLAS Pathology is an AI powered platform that helps pharmaceutical companies test new drugs for safety by replacing fragmented workflows with a single, collaborative system for study management, image analysis, and AI-assisted decision support.
My role was to design the end-to-end UX of the platform while balancing scientific rigor, regulatory compliance, and day-to-day usability for multiple user roles.
At a Glance
Enterprise, role-based digital pathology platform designed to replace fragmented lab workflows with a single, compliant system of record.
Reduced study setup time from ~2 days to ~4 hours
Cut manual data-entry errors by ~90%
Enabled GLP & FDA 21 CFR Part 11 compliance without interrupting user flow
Designed role-based experiences for Study Directors, Pathologists, and Technicians
Helped reposition the product from a tool to a workflow platform
The problem
One Study, 2,000 Slides & "Zero" Connection!
In most pathology labs, reviewing a single study required juggling:
Excel spreadsheets (metadata, animal IDs, dose groups)
File systems with hundreds of cryptically named image files
Legacy slide viewers with no awareness of study context
Pathologists were spending 30% of their day just matching files to spreadsheets.
A single typo while mapping a slide to an animal could:
Trigger compliance flags
Invalidate a study
Delay regulatory submissions by weeks
This constant context switching created cognitive fatigue, slowed reviews, and increased risk.
Research & Discovery
Research Methods
Stakeholder interviews with pathologists, study directors, and lab technicians
Workflow walkthroughs of existing Excel + file-system setups
Artifact analysis (Excel sheets, folder structures, SOPs)
Competitive analysis of digital pathology tools
Domain research on GLP, 21 CFR Part 11, and audit requirements
Early usability tests with low-fidelity flows
Key Insight
The core problem wasn’t image viewing, it was
context management.
Images, metadata, annotations, and decisions lived in different places, forcing users to mentally stitch information together.
Reframing the challenge
Instead of asking “How do we digitize pathology?”
I reframed the problem around workflow integrity.

Strategy
Old mental model:
Folders → Files → Spreadsheets
New mental model:
Study → Animal → Tissue → Slide → Insight
This shift informed:
Navigation structure
Search behavior
Metadata relationships
Permissions and collaboration rules
User Archetype
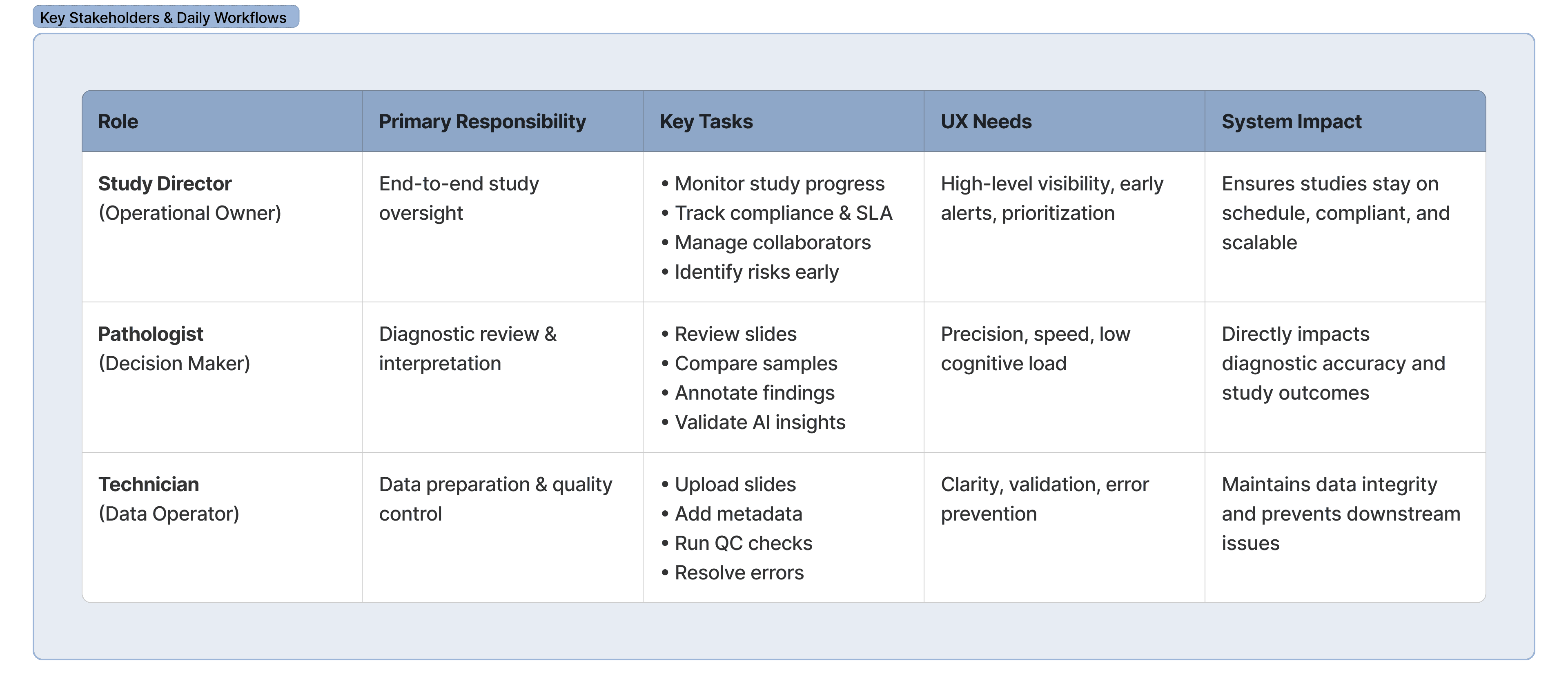
Information Architecture
The platform is built on a shared data model, but the interface adapts by role ensuring each user sees only what’s relevant to their responsibilities while staying aligned on the same study.
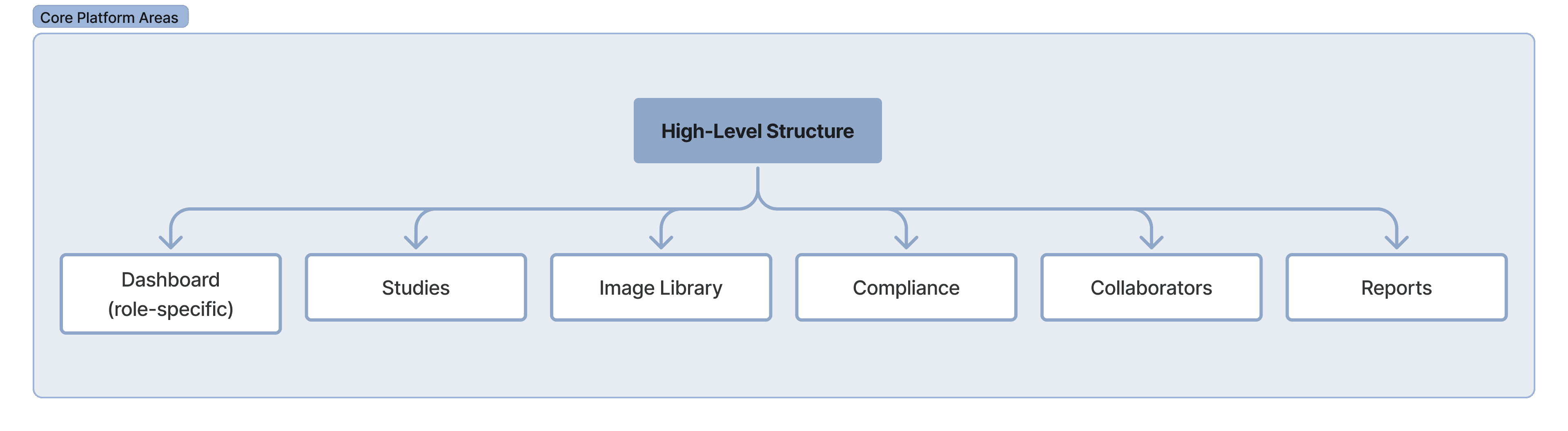
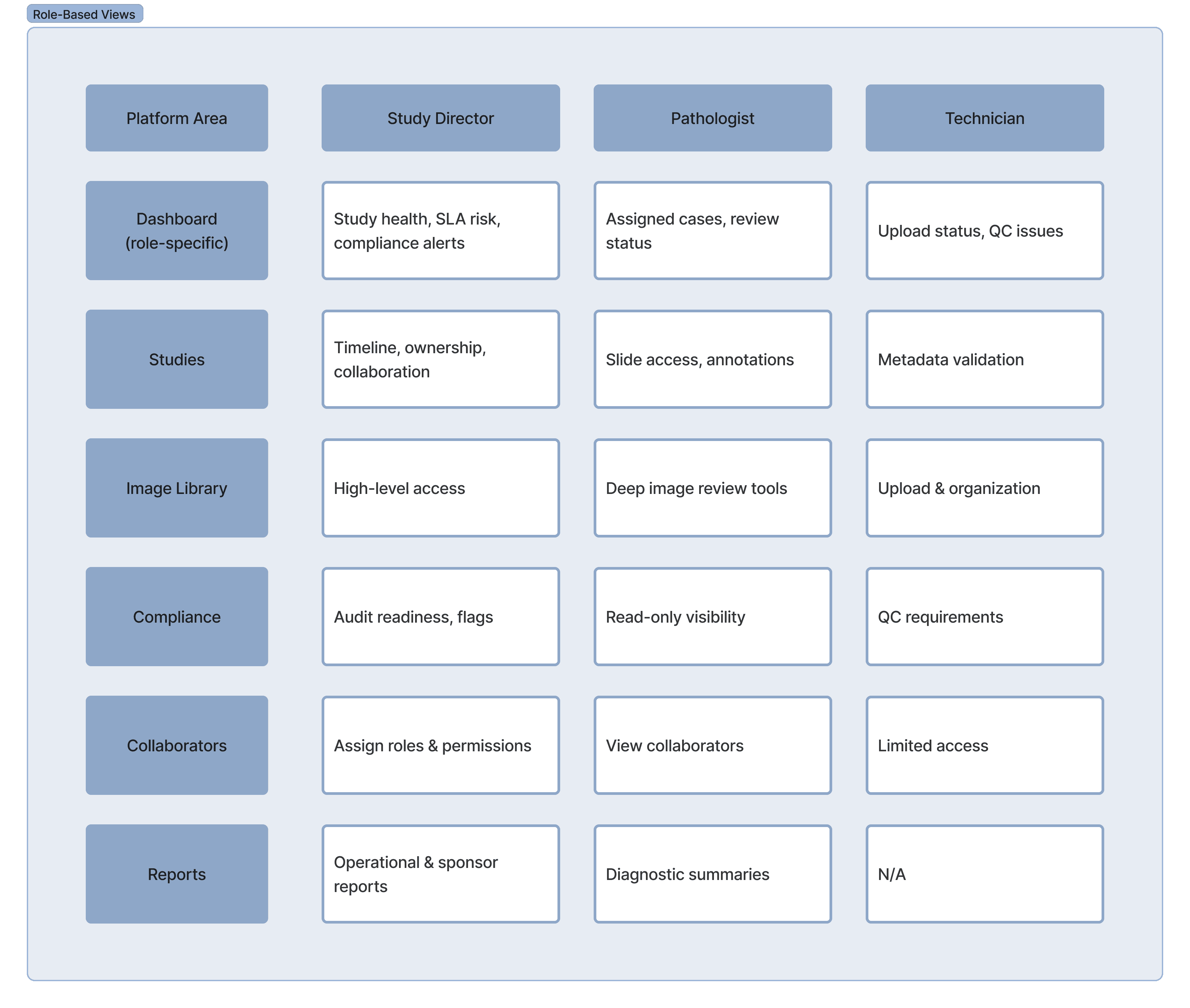
Why This Matters?
This approach avoided a one-size-fits-all interface while maintaining a single source of truth. Each role interacts with the same study data, but through an interface optimized for their decisions and workflows.
Design Decision
User problem
Study setup involved manually mapping thousands of slides to animals and dose groups using Excel.
Proposed solution
A visual, drag-and-drop mapping wizard that replaced typing with spatial reasoning.
Batch cards represent barcode ranges
Drop zones represent biological groupings
Real-time validation prevents invalid mappings
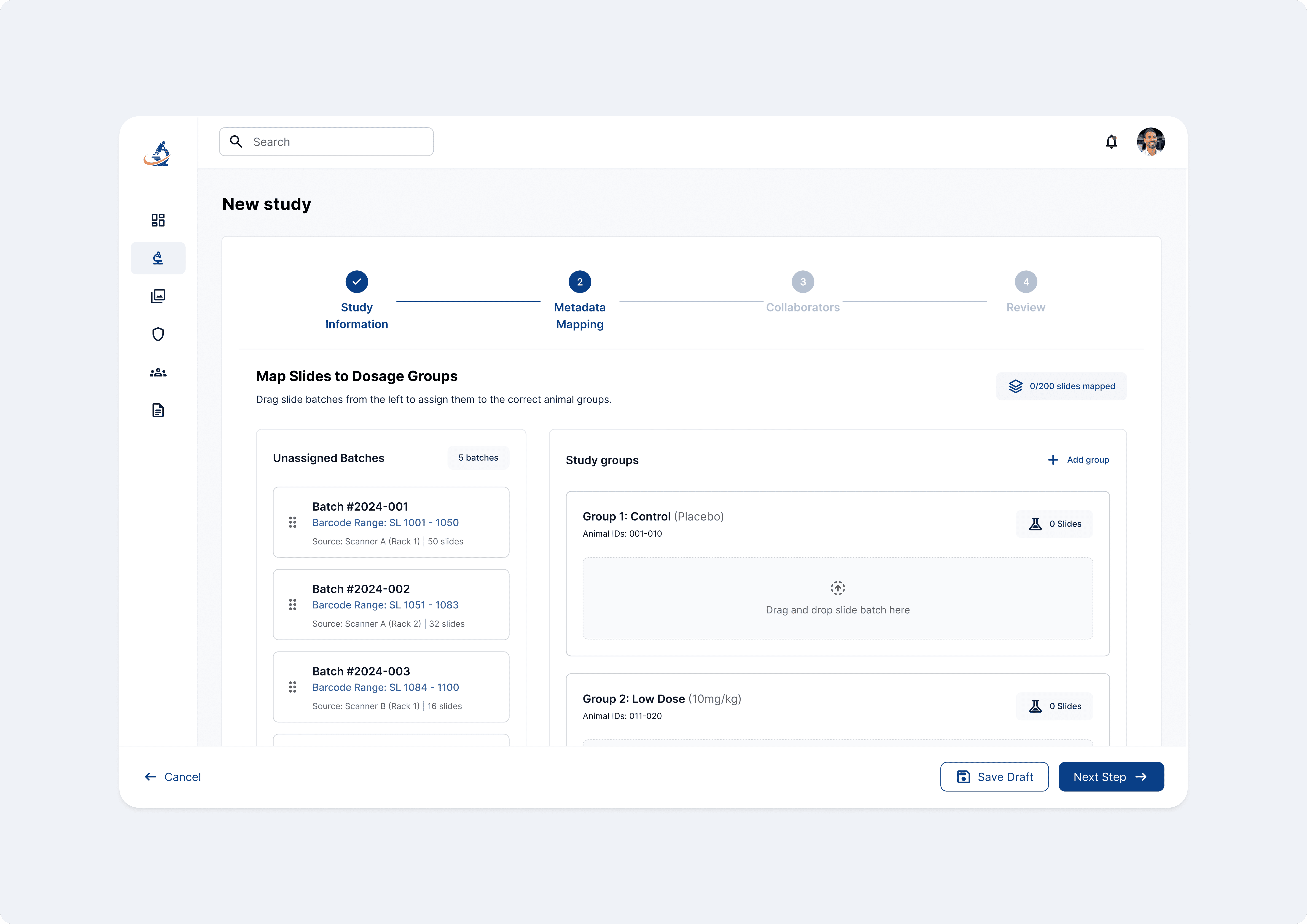
Design Decision
User problem
Study setup involved manually mapping thousands of slides to animals and dose groups using Excel.
Proposed solution
A visual, drag-and-drop mapping wizard that replaced typing with spatial reasoning.
Batch cards represent barcode ranges
Drop zones represent biological groupings
Real-time validation prevents invalid mappings

I design. You need stuff designed.
Coincidence?
I think not.
I’m currently open to Full-Time Opportunities. Let’s make scroll-stopping magic together. 👩🏻💻🪄


