ATLAS Pathology is a digital pathology and image-analysis platform serving preclinical research and life sciences organizations operating under GLP and FDA 21 CFR Part 11 requirements.
The company’s mission is to help labs reduce turnaround time, improve data integrity, and scale pathology workflows, by embedding AI-assisted analysis into compliant, end-to-end systems rather than selling standalone AI algorithms.
THE CLIENT
Atlas Pathology (name changed under NDA)
Date
Founding Product Designer
Industry
8 months
Scope of work
Enterprise SaaS
Product Design
Web App Tool
I worked as the sole Product Designer, partnering closely with product, engineering, and QA.
Scope of work
User research & workflow analysis
Information architecture
Interaction & visual design
Prototyping & usability testing
Design system alignment
Product strategy support
This was a live commercial product. [Name changed under NDA]
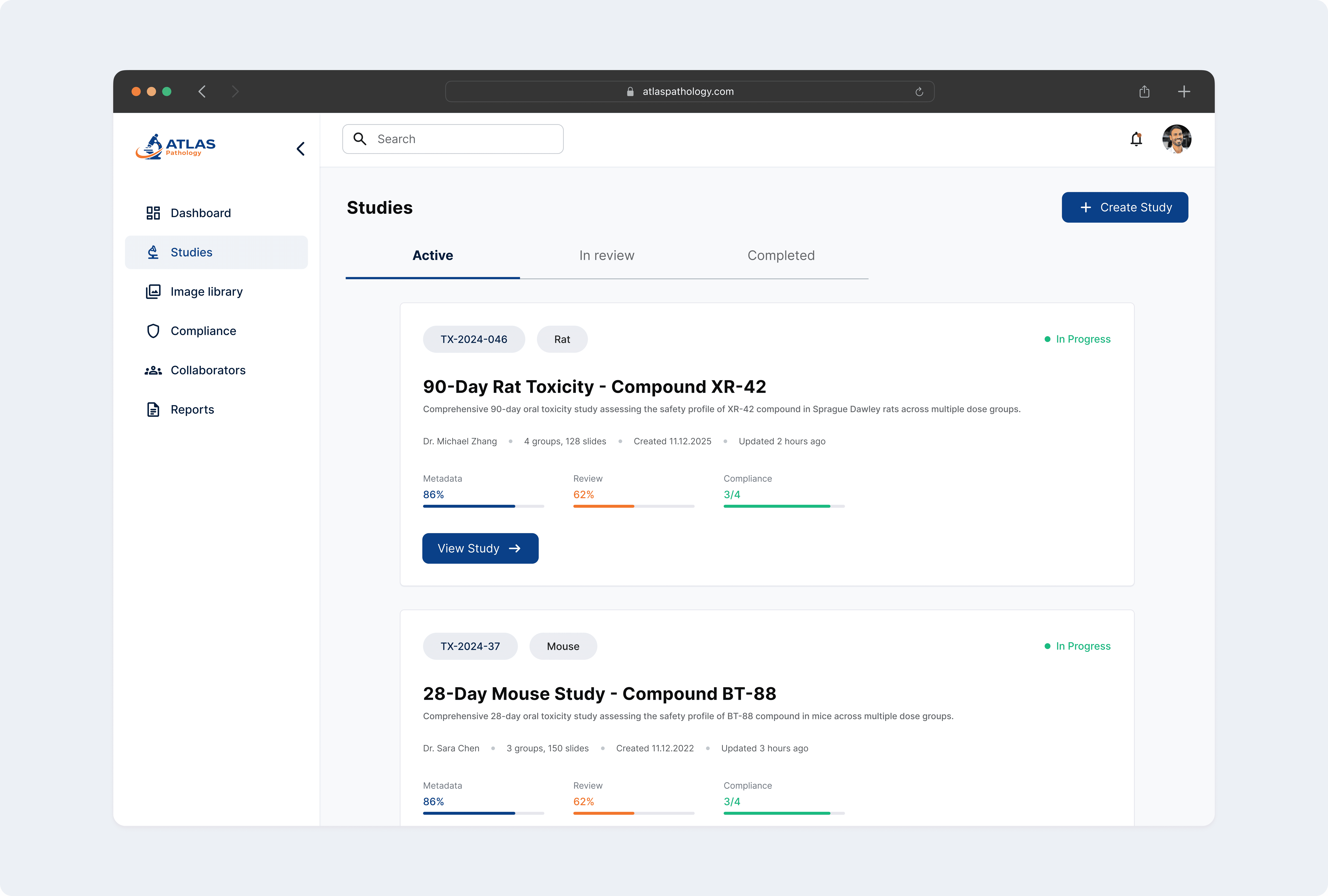
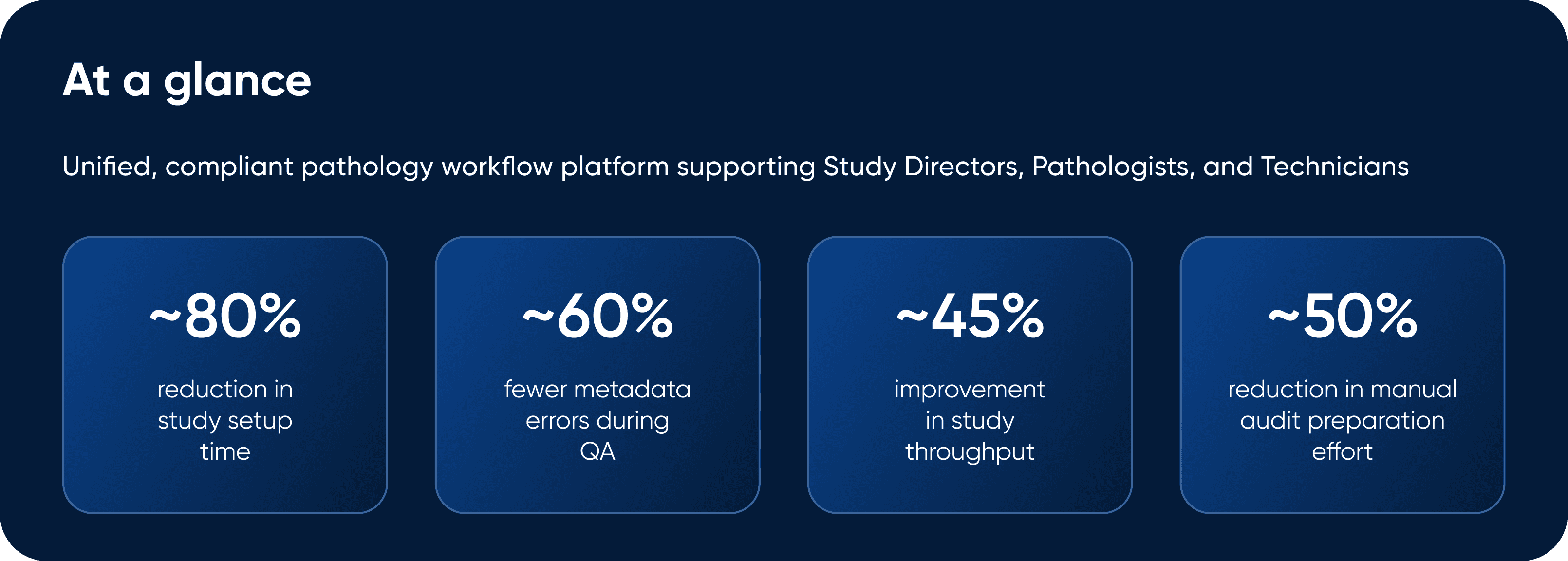
One Study, 2,000 Slides & "Zero" Connection!
In most pathology labs, reviewing a single study required juggling:

Research Methods
Interviews with Study Directors, Pathologists, and Technicians
Workflow walkthroughs of study setup and review
Analysis of existing spreadsheets, folder structures, and SOPs
Competitive review of digital pathology tools
Early usability testing with low-fidelity flows
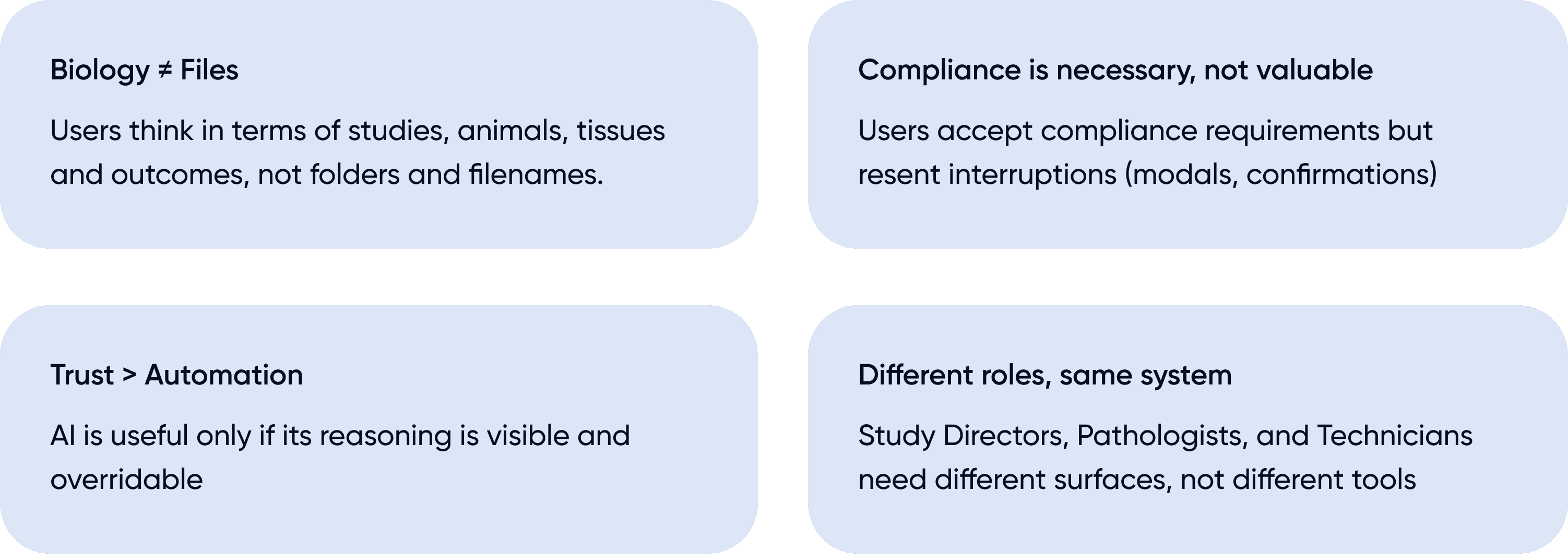

Reframing the challenge
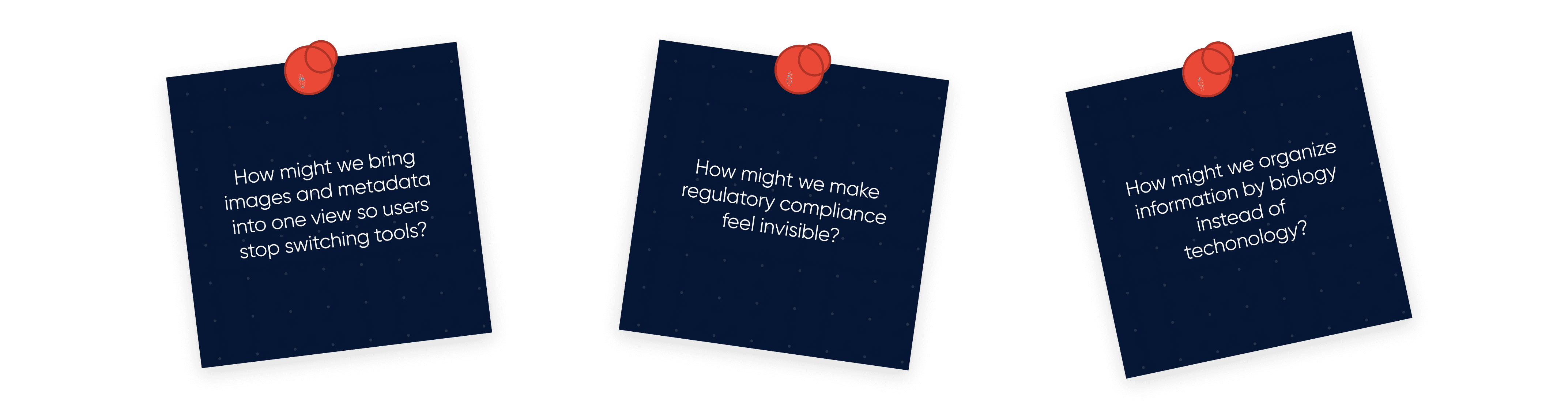
Design Decision
User problem #1
Technicians manually mapped thousands of slides using Excel which was slow and error-prone.
Proposed solution
Design a visual mapping wizard to guide study setup and reduce manual data-entry errors.
Batch cards represent barcode ranges
Drop zones represent biological groupings
User problem #2
FDA 21 CFR Part 11 requires logging every meaningful action, but modal confirmations break focus.
Proposed solution
Design compliance workflows that are continuously enforced without requiring explicit user intervention.
Silent audit logging in the background
Dedicated compliance dashboard for governance
Clear separation between: Operational alerts (main dashboard) and Compliance oversight (compliance dashboard)
User problem #3
While AI models could surface potential patterns and regions of interest, pathologists were hesitant to rely on them.
Proposed solution
Introduce AI as a transparent decision-support layer that assists pathologists without replacing clinical judgment.
Designed AI as transparent decision support, not automated decision-making
Introduced confidence indicators to communicate uncertainty instead of absolute predictions
Used visual overlays mapped directly to tissue regions to make AI reasoning inspectable
Preserved human authority with clear options to confirm, question, or override AI findings
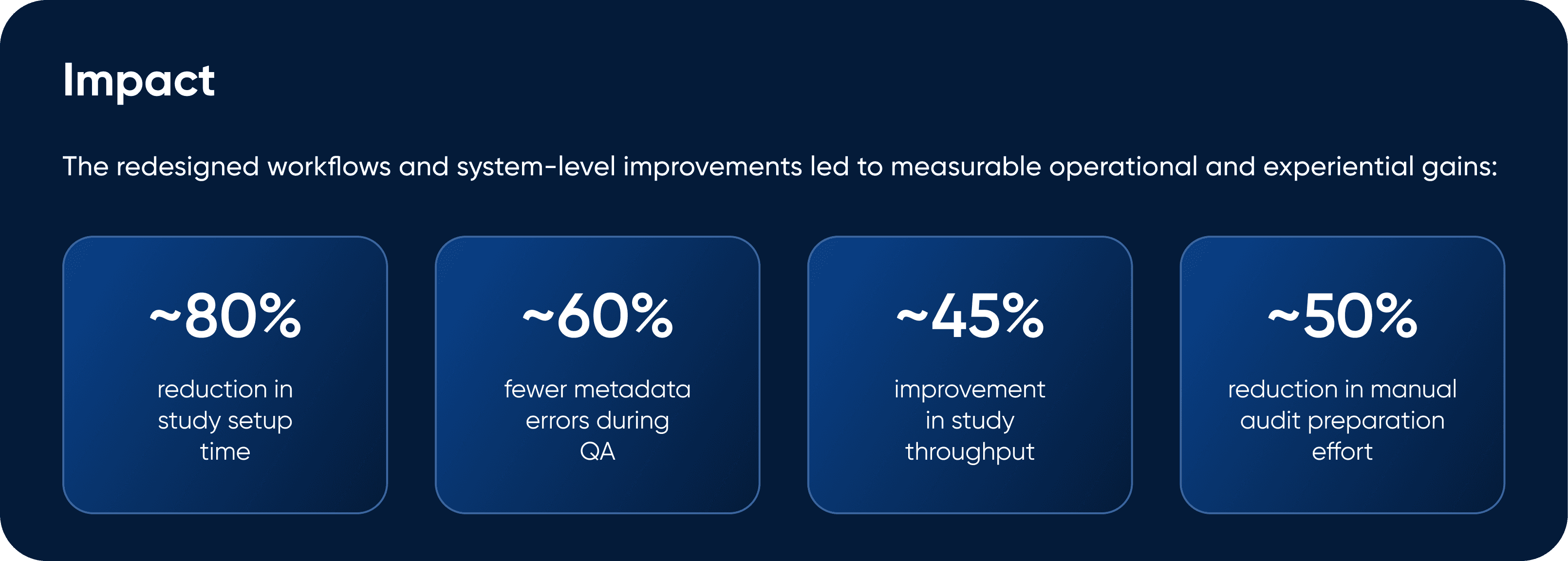
Reflections & Learnings
This project reinforced the importance of sequencing in complex systems addressing foundational workflow, data integrity, and compliance challenges before introducing advanced capabilities like AI.
Designing for regulated environments required balancing usability with accountability, and ensuring that trust was built into the system rather than added on top.




